 Anion Recognition
Anion Recognition

The field of anion recognition and sensing by synthetic molecular receptors has become one of the most important areas in supramolecular chemistry due to the important role that anions play in numerous biological and environmental processes. Despite the enormous progress achieved in this field, selective anion recognition continues to be a real challenge, especially in water and biological media due to the intrinsic characteristics of anions. Motivated by the high interest in the recognition and detection of anions, our research groups have developed different molecular receptor/sensor to reach this objective in the last years. Specifically we have used the 1,2,3-triazole ring or 1,2,3-triazolium ring as binding site in the design of new anion receptors. The collective electronegativity of the three nitrogen atoms polarises the C-H bond, which, in combination with the lone electron pairs on the nitrogen atoms, acts to establish a large 5D dipole oriented along the C–H bond, with its positive end directed almost in line with the C5–H bond. Futhermore, we have also used the imidazolium cations, as anion binding sites, siformed by substitution at nitrogen of imidazole. This novel type of charged hydrogen bonding has the advantage of having both electrostatics and hydrogen bonding interactions in the same binding site, allowing the possibility of pH-independent binding compared with other, more conventional N-H-based hydrogen bond donors.
Selected Publications:
Synthesis, Structure and Anion Sensing Properties of a Dicationic Bis(imidazolium)-Based Cyclophane.
Paula Sabater; Fabiola Zapata; Antonio Caballero; Ibon Alkorta; Carmen Ramirez de Arellano; Jose Elguero; Pedro Molina.
ChemistrySelect. 2018, 3, 3855 - 3859.
DOI: 10.1002/slct.201800809
Anion Recognition Strategies Based on Combined Noncovalent Interactions.
Pedro Molina; Fabiola Zapata; Antonio Caballero.
Chem. Rev., 2017, 117, 9907-9972.
DOI: 10.1021/acs.chemrev.6b00814
2,4,5-Trimethylimidazolium Scaffold for Anion Recognition Receptors Acting Through Charge-Assisted Aliphatic and Aromatic C−H Interactions
Paula Sabater; Fabiola Zapata; Antonio Caballero; Israel Fernández; Carmen Ramirez de Arellano; Pedro Molina
J.Org. Chem., 2016, 81, 3790-3798.
DOI: 10.1021/acs.joc.6b00468
 Halogen Bonding
Halogen Bonding
One exciting alternative to the traditional non-covalent interactions could be the utilization of the halogen bonding in the design of new anion sensors. The term halogen bonding (XB) is used in analogy with the well known hydrogen bonding (HB) and is the noncovalent bonding interaction between halogen atoms that function as electrophilic centres (Lewis acids) and neutral or anionic Lewis bases. The origin of the attraction is attributed to a positive region on the halogen that corresponds to the electronically-depleted outer lobe of the R-X bond. The resulting positive electrostatic potential lies on the surface of the halogen, located at the terminus of the R-X axis while a band of negative charge remains around the equator of the halogen atom. The intermolecular force known as halogen bonding arises from the interaction of the positively charged sigma-hole with electron donating species, resulting in a strongly linear geometry which maximises the interface of opposite charges.
To date, most of the investigations into halogen bonding have been conducted in the solid state where the non-covalent interaction has been imaginatively exploited in the crystal engineering of magnetic, conducting and liquid crystalline materials. In the last years the study of the halogen bonding in the solution phase has growing enormously due to the analogy to ubiquitous hydrogen bonding.
Selected Publications:
Ferrocene–Triazole Combination as a Benchmark for the Electrochemical Detection of Noncovalent Halogen-Bonding Interactions.
Fabiola Zapata; Antonio Caballero; Pedro Molina.
Eur. J. Inorg. Chem. 2017, 237-241.
DOI: ejic.201600838
Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions.
Fabiola Zapata; Sergio J. Benítez-Benítez; Paula Sabater, Antonio Caballero; Pedro Molina.
Molecules. 2017, 2273.
DOI: 10.3390/molecules22122273
Comparative Study of Charge-Assisted Hydrogen- and Halogen- Bonding Capabilities in Solution of
Two-Armed Imidazolium Receptors toward Oxoanions.
Paula Sabater; Fabiola Zapata; Antonio Caballero; Néstor de la Visitación; Ibón Alkorta; José Elguero; Pedro Molina.
J. Org. Chem., 2016, 81, 7448-7458.
DOI: 10.1021/acs.joc.6b01146
Host-Guest Chemistry: Oxoanion Recognition Based on Combined Charge-Assisted C-H or
Halogen-Bonding Interactions and Anion•••Anion Interactions Mediated by Hydrogen Bonds.
Lidia González; Fabiola Zapata; Antonio Caballero; Pedro Molina; Carmen Ramirez de Arellano; Ibon Alkorta; Jose Elguero.
Chem. Eur. J., 2016, 22, 7533-7544
DOI: 10.1002/chem.201600379
Comparative Study of Charge-Assisted Hydrogen- and Halogen- Bonding Capabilities in Solution of
Two-Armed Imidazolium Receptors toward Oxoanions.
Paula Sabater; Fabiola Zapata; Antonio Caballero; Néstor de la Visitación; Ibón Alkorta; José Elguero; Pedro Molina.
J. Org. Chem., 2016, 81, 7448-7458.
DOI: 10.1021/acs.joc.6b01146
 Chalcogen Bonding
Chalcogen Bonding

Chalcogen bonding (ChB) is a subgroup of the σ-hole family that originates from the interaction between an electron rich atom or group of atoms and the σ-hole of an element belonging to group 16. In fact, chalcogen bonding has been recently defined by a IUPAC commission as “net attractive interaction between an electrophilic region associated with a chalcogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity”. The ChB interaction is more directional than hydrogen bonding (HB) and comparable to her sister halogen bonding (XB), however the presence of two σ-holes (opposite to both covalent bonds) confers to this interaction more possibilities of binding and fine tuning of the σ-holes individually. Compared to HB, both ChB and XB interactions have greater hydrophobicity and they are less sensitive to solvent effects and pH. The binding strength of ChB is comparable to the ubiquitous HB and its high directionality allows greater precision in three dimensional spatial control. In fact, these distinctive characteristics of ChBs have been used in crystal engineering, pharmaceutics, catalysis, self‐assembly processes and materials design. However, studies of ChB interactions in solution remain scarce.
Selected Publications:
Anion recognition by neutral chalcogen bonding receptors: experimental and theoretical investigation
Encarnación Navarro-García, Bartomeu Galmés, María D. Velasco, Antonio Frontera and Antonio Caballero
Chem. Eur. J., 2020, 26, 4706-4713
DOI: 10.1002/chem.201905786
 Supramolecular Polymers
Supramolecular Polymers

Supramolecular polymers represent a novel class of macromolecules, in which the self-assembly serves as a powerful tool and keeps the monomer units together through reversible non-covalent bonds. The dynamic and reversible nature of non-covalent interactions provides an elegant and interdisciplinary combination of supramolecular chemistry and polymer science. A variety of non-covalent interactions, such as multiple hydrogen bonding, hydrophobic interactions, pi-pi stacking, metal-ligand coordination, integrated non-covalent interactions, and an elegant combination of pi-pi and hydrogen bonding interactions have been widely served as the non-covalent driving forces for controlled supramolecular polymerization processes. The interaction of molecules by non-covalent forces generates supramolecular polymers that can exhibit properties (optical, chiroptical, etc.) not presented by isolated block molecules. Our research group has described recently a new class of supramolecular polymer, where the design is based on the hypothesis that polydentate halogen-bonding anion receptors could form a supramolecular polymer by forming consecutive intermolecular anion complexes through non-covalent host-guest halogen bonding interactions preferably to the anion recognition by a simple receptor. In this way it is possible to obtain self-assembled systems where each monomer is linked to another by an anion by halogen bonding interactions, resulting in a high degree of polymerization in solution
Selected Publications:
Self-Assembly Supramolecular Polymer by Anti-Electrostatic Anion-Anion and Halogen Bonding Interactions
Fabiola Zapata, Lidia Gonzalez, Adolfo Bastida Delia Bautista and Antonio Caballero
Chemical Communications 2020, 56, 7084-7087
DOI: 10.1039/D0CC02831B
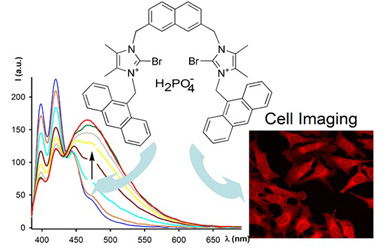
Selective fluorescence sensing of H2PO4− by the anion induced formation of self-assembled supramolecular polymers
Paula Sabater, Fabiola Zapata, Adolfo Bastida and Antonio Caballero
Org. Biomol. Chem., 2020, 18, 3858-3866
DOI: 10.1039/d0ob00258e
Interlocked Supramolecular Polymers Created by Combination of Halogen- and Hydrogen-Bonding Interactions through AnionTemplate Self-Assembly.
Fabiola Zapata; Lidia Gonzalez; Antonio Caballero; Delia Bautista; Adolfo Bastida; Pedro Molina.
J. Am. Chem. Soc., 2018, 140, 2041-2045.
DOI: 10.1021/jacs.7b12612
 Español
Español English
English